Comorbidity profile assessment in patients with psoriatic arthritis: A comprehensive study
Summary. Psoriatic arthritis (PsA) is a persistent autoimmune type of arthritis marked by inflammation in the joints often associated with psoriasis, elevates the risk of various concomitant disorders, notably cardiovascular, endocrine, and psychological comorbidities; thus, comprehending the intricate relationship between psoriatic arthropathy and these comorbidities is paramount for optimizing diagnostic, therapeutic, and management approaches for patients with PsA. This study aimed to investigate the prevalence and impact of comorbidities in PsA patients. A total of 223 patients were examined from 2018–2023 at Ternopil Regional Hospital’s rheumatology department, following a comprehensive diagnostic protocol. Comorbidity indexes were used to evaluate the impact of comorbidities on 10-year survival in those patients. The study revealed that 55.6% of patients with psoriatic arthritis had cardiovascular diseases, 37.7% suffered from endocrine disorders, and 75.8% of patients reported a decline in the quality of life in the categories of physical activity, pain, and mental health over the past 5 years. The findings reveal individuals with PsA have a moderate risk of experiencing unfavorable cardiovascular events over the next decade, highlighting the need for effective cardiovascular risk management. This study underscores the significance of addressing cardiometabolic comorbidities, with a particular emphasis on cardiovascular disorders, to mitigate long-term risks and improve patient well-being.
DOI: 10.32471/rheumatology.2707-6970.93.18212
UDC 616-06:616.72-002-02:616.517-07
INTRODUCTION
Psoriatic arthritis (PsA) is a complex autoimmune inflammatory arthritis condition often linked with psoriasis. Belonging to the spondyloarthropathy group, PsA affects both the spine and peripheral joints, often accompanied by enthesitis and dactylitis [1, 2]. Aside from the skin and joint symptoms, psoriatic arthritis is characterized by a range of additional immune-mediated manifestations outside the joints and skin. These may encompass conditions such as inflammatory bowel disease and autoimmune eye disorders. Moreover, according to studies conducted by S. Gupta et al. [3], patients commonly experience comorbidities, which can significantly impact disease domains such as activity, impact on patients, functional abilities, and overall quality of life.
Based on research conducted by A. Haddad et al. [4], O. Elalouf et al. [5], the mortality rate in individuals with PsA surpasses that of the general population, primarily due to cardiovascular (CV) diseases. Recently, in their study, L.V. Khimion & A.V. Boiko [6] confirmed well-established data that systemic inflammation triggered by psoriatic disease results in heightened insulin resistance, endothelial cell dysfunction, atherosclerosis, and atherothrombosis, culminating in serious adverse conditions. Endothelial cell dysfunction (ED) and inflammation initiate atherosclerosis development, correlating with the disease course and insulin resistance, as indicated by studies conducted by M. Marushchak et al. [7] and F.C.A. Babalic et al. [8].
The cumulative effect of inflammation correlates with the severity of atherosclerotic plaques in individuals affected by psoriatic arthritis, as highlighted in a study conducted by L.M. Perez-Chada & J.F. Merola [9], indicating that PsA is linked to a 55% higher likelihood of developing cardiovascular conditions, such as ischemic heart disease (IHD), cerebrovascular disease, and congestive heart failure.
Psoriatic arthropathy is associated with a heightened occurrence of type 2 diabetes mellitus, obesity, and metabolic syndrome in contrast to both the general population and the rheumatologic profile. Notably, there is an independent association between insulin resistance and the severity and duration of the disease [3, 8, 9]. Additionally, these patients exhibit an increased prevalence of non-alcoholic fatty liver disease (NAFLD), which is further associated with hypercholesterolemia, hypertriglyceridemia, obesity, metabolic syndrome, and disease severity. It is worth noting that medications such as nonsteroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), and tumor necrosis factor-alpha (TNF-α) blockers could also contribute to hepatotoxicity and abnormalities in liver function tests [3, 10].
One of the largest studies was conducted in 2022 by the Framingham Heart Study team (Framingham, USA), led by B. Tejada et al. [11] of the National Heart, Lung, and Blood Institute, where 7,287 patients were examined. Up to 26 proteins associated with the inflammatory process were assessed, and higher levels of inflammation were found to be associated not only with abnormal levels of lipids, glucose, fibrinogen, uric acid but also with an increased risk of developing hypertension and diabetes.
Building upon the high prevalence of comorbid pathology and recognizing the substantial negative impact it exerts on the course of the primary ailment, as described above, recommendations for its management have been even integrated into the core global treatment guidelines. In June 2022, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) released updated guidelines, with the previous version dating back to 2015. This comprehensive framework not only addresses the treatment of peripheral and axial arthritis, enthesitis, dactylitis, and skin and nail psoriasis but also offers essential insights into managing comorbidities that profoundly influence the treatment strategy [12].
Psoriatic arthropathy is a complex autoimmune inflammatory condition that not only affects joints and skin but also brings forth a range of systemic complications. Among these, CV diseases, obesity, metabolic syndrome, type 2 diabetes, osteoporosis, fatty liver disease, depression, and anxiety have emerged as significant comorbidities in those patients. The incidence of these comorbid conditions is significantly elevated in PsA in comparison to the general population. Furthermore, these comorbidities not only complicate the clinical course of this nosology but also contribute to increased morbidity and mortality rates among affected individuals. Understanding the intricate relationships between PsA and its comorbidities is of paramount importance for improving patient care, enhancing treatment strategies, and ultimately, enhancing the quality of life for those living with this disese. The aim of this study was to investigate the prevalence and impact of comorbidities in patients with PsA.
MATERIALS AND METHODS
The study included two hundred twenty-three patients with PsA who were examined at the rheumatology department of Ternopil University Hospital from 2018 to 2023. Participants were required to be at least 18 years old, have a minimum of 6 months of follow-up during the study visit, and fulfill the disease criteria based on the CASPAR criteria [2, 13]. All patients in the study exhibited chronic plaque psoriasis and reported enduring discomfort or inflammation in any of their fingers (toes) and/or sacroiliitis. Individuals with a history of inflammatory bowel diseases, or reactive arthritis associated with urogenital, intestinal, or other forms of infections (such as Borrelia burgdorferi or viral etiology) were excluded from the study.
All patients who agreed to participate underwent a comprehensive evaluation using standard diagnostic methods. This included common blood tests with erythrocyte sedimentation rate (ESR) and thrombocyte counts, urinalysis, assessment of acute phase indicators such as C-reactive protein (CRP), lipid profile (including plasma lipoprotein particles such as total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides), X-ray or magnetic resonance imaging (MRI) of the affected joints (including sacroiliac joints for differentiation of active/inactive sacroiliitis), electrocardiogram (ECG), echocardiography, ultrasound examination with checking for thickening of the intima-media of the common (TCIM) carotid artery, and detection of Human Leukocyte Antigen B27 (HLA B-27), thyroid function tests, bone profile, hepatitis markers, Rheumatoid factor, anti-nuclear antibodies (ANA) and anti-cyclic citrullinated peptide antibodies (anti-CCP).
To evaluate the 10-year risk of CV disease, both the QRISK-3 and systematic coronary risk evaluation (SCORE) scales were employed [14–16]. Disease activity was assessed using the Disease Activity Score for Psoriatic Arthritis (DAPSA), where a score <4 indicated remission, >4–<14 represented low disease activity, >14–<28 indicated moderate disease activity, and >28 signified high disease activity [2]. The extent of functional ability and patients’ quality of life was measured using the Health Assessment Questionnaire-Disability Index (HAQ-DI) and the Sf-36 [17–19]. Skin involvement in the study was evaluated using the Psoriasis Area and Severity Index (PASI), a well-established tool for assessing psoriasis severity. A score below 1 indicated remission, while a score between 1 and 10 was categorized as mild, 10–20 as moderate, and a score above 20 as severe psoriasis [2;18].
The Charlson Comorbidity Index (CCI) and PsA-comorbidity index (PsACI) were employed to evaluate the comorbidity profile and estimate the 10-year survival rate among PsA patients [20–22]. The PsACI was developed through a multivariate regression analysis, identifying a total of 29 comorbidities. Based on the regression coefficients of these variables, a scoring system was established, resulting in a score range from 0 to 37. The scores of each comorbidity are detailed in Table 1, a cut-off point of 8 was determined, which exhibited a sensitivity of 97.5% and a specificity of 87% [21; 23].
Table 1. Scores for Each Comorbidity
| PsACI | |
|---|---|
| 29 Comorbidities | Assigned Score 0-37 |
| Disease Severity score > 3 | 5 |
| Metabolic Syndrome | 3 |
| Myocardial Infarction | 2 |
| Ischemic Heart Disease | 2 |
| Depression | 2 |
| Diabetes Mellitus | 2 |
| Fracture | 2 |
| Hypertension | 1 |
| Hyperlipidaemia | 1 |
| Arrhythmia | 1 |
| Cerebrovascular Disease | 1 |
| Peripheral vascular disease | 1 |
| Liver disease | 1 |
| Fall | 1 |
| 29 Comorbidities | Assigned Score 0-37 |
| Pulmonary disease | 1 |
| Endocrine | 1 |
| GIT | 1 |
| Renal | 1 |
| Tumour | 1 |
| Infection | 1 |
| Anxiety | 1 |
| Smoking | 1 |
| Osteoporosis | 1 |
| Periodontitis | 0.5 |
| Osteoarthritis | 0.5 |
| Fibromyalgia | 0.5 |
| Eye inflammation/ uveitis | 0.5 |
| Vasculitis | 0.5 |
| Amyloidosis | 0.5 |
With the aim of assessing the treatment impact on the comorbidity profile in the examined patient cohort, individuals with a disease duration exceeding 10 years and with moderate to high disease activity were selected, either historically or at the time of examination, accounting for 198 cases (88.8%). The first group (n=46) consisted of patients who did not receive any treatment or only received symptomatic NSAIDs. The second group (n=124) comprised patients who were treated with conventional synthetic disease-modifying antirheumatic drugs (DMARDs), while the third group (n=28) underwent therapy with biological agents (primarily golimumab in 75% of cases) in combination with conventional synthetic DMARDs.
For statistical analysis, traditional methods of analysis were employed. The data were analyzed using IBM SPSS Statistics 27, a statistical software platform, to assess and compare the various parameters among the study participants. Statistic comparisons for normally distributed continuous variables were performed with the Student t-test and Mann–Whitney U test for nonnormally. Spearmans rank correlation coefficient was used to calculate the correlations. To identify indicators influenced by the type of prescribed treatment, parametric analysis of variance and rank-based analysis of variance (using Kruskal-Wallis) were employed. When significant intergroup differences were detected (p<0.05), a post-hoc test was conducted for parametric analysis of variance, involving pairwise group comparisons using the Tukey criterion, and a comparison of mean rank values was performed for non-parametric analysis of variance. Participants provided informed consent, and their confidentiality and anonymity were maintained. The study had no adverse effects on participants’ health and adhered to the ethical norms of the Helsinki Declaration for research involving human participants [24].
RESULTS AND DISCUSSION
The average age of individuals in the study stood at 39.8±0.74 years, coupled with a mean disease duration of 15.8±0.62 years. Isolated psoriatic spondylitis was diagnosed in 35 patients (15.7%), while another 111 (49.8%) exhibited it alongside peripheral arthritis, and 77 (34.5%) solely displayed arthritis, manifesting in various clinical forms (mono-oligo or polyarthritis).
The evaluation of PsA progression utilizing the DAPSA index unveiled a prevalence of high (43.5%) and moderate (48.4%) disease activity levels, with scarce instances of individuals showing low (8.1%) disease activity or reaching a state of remission (0%). Concerning the scrutiny of the clinical manifestations of psoriatic severity as per the PASI scale, 68 patients (30.5%) showcased a severe course, 118 patients (52.9%) demonstrated a moderate trajectory, and 37 patients (16.6%) displayed a milder course, respectively. The analysis conducted to assess the overall well-being and quality of life of patients (based on the Sf-36 scale) revealed that over the past 5 years, 169 individuals (75.8%) noted deterioration in the following categories: physical functioning, bodily pain, and mental health. Health assessment (using HAQ-DI) demonstrated the presence of a moderate to severe level of impairment in the majority of cases (1.85±0.11).
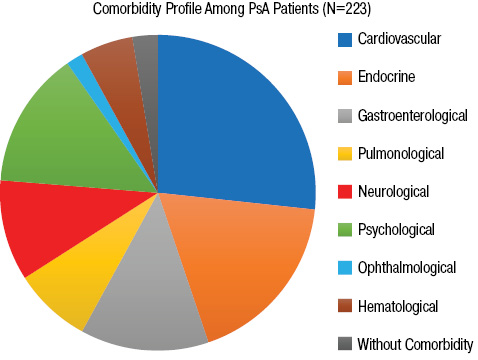
Analysis of 223 patients (Fig. 1) unveiled a substantial inclination towards cardiometabolic (CM) comorbidities in PsA. Among the cases, 55.6% (124 patients) displayed CV disorders, while 37.7% (84 patients) exhibited endocrine disorders encompassing metabolic irregularities. Psychological conditions, notably depression and anxiety, were noticed in 29.2% (65 patients), followed by gastroenterological ailments in 27.4% (61 patients), neurological conditions in 21.5% (48 patients), pulmonological issues in 16.6% (37 patients), hematological problems in 11.2% (25 patients, primarily anemia), and autoimmune eye diseases in 3.6% (8 patients, primarily uveitis). Remarkably, during the examination, 119 patients (53.4% of cases) presented mixed pathologies, signifying the coexistence of two or more nosologies, whereas only 5.4% (12 patients) exhibited no indications of any other ailment besides psoriasis and PsA.
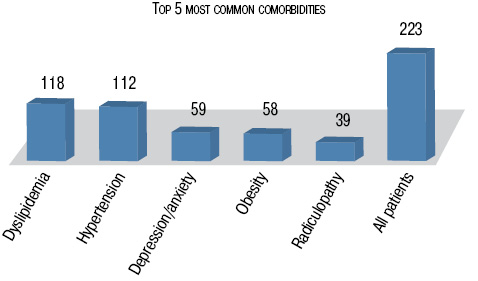
Among the observed comorbidities, dyslipidemia was among the most frequently noted, occurring in 118 patients (53.0%). Similarly, hypertension was prevalent in 112 patients (50.2%), followed by depression and/or anxiety affecting 59 patients (26.5%), obesity in 58 patients (26.0%), and radiculopathy in 39 patients (17.5%) (Fig. 2).
To gauge comorbidity indexes, CCI, a well-recognized and frequently employed measure of comorbidity. It averaged at 1.58 points (95% CI 0.0–5.0), suggesting a 10-year survival rate of approximately 93% among PsA patients. Furthermore, PsACI, a distinct index tailored for PsA patients. It accounts for the severity stage of PsA and encompasses a broader spectrum of diseases in its algorithmic process. The PsACI score registered at 8.85 points (95% CI 0.5–15.5), potentially aiding in identifying subsets of PsA patients at elevated risk for comorbidities. The findings indicated a higher prevalence of comorbidities associated with heightened PsA disease activity, duration, and functional constraints. A threshold of 8 was pinpointed in 142 instances (63.7%), facilitating the recognition of patients facing a heightened 10-year mortality risk.
The study also delved into CV risk assessment in PsA patients, as heart and vascular conditions constituted a significant portion of the comorbidity profile in examined individuals. It was ascertained that psoriatic arthropathy is linked to an intermediate (9.4±0.35%) likelihood of encountering adverse heart-related events within the following 10 years. This stands 8 to 9 times higher than the risk for healthy age, sex, and ethnicity-matched subjects. Patients who did not receive DMARD treatment during a prolonged disease course had an increased likelihood of experiencing significant adverse cardiovascular events (11.9±0.47%). This association remained independent of conventional CV risk factors and correlated with markers of disease severity and activity, underscoring the critical role of optimal PsA treatment in enhancing CV outcomes.
Conversely, the risk appraised as low (1.9±0.24%) using the classical SCORE. Additionally, according to requirements, this scale was applied only to individuals over 40 years old, as well as the absence of accounting for the influence of other important risk factors in the algorithm, e.g. in this case, the systemic inflammatory process, which, alongside this, prevented its further use in this study. Moving forward to the statistical analysis, this study aimed to uncover the factors affected by the prescribed treatment types. These analyses allowed us to identify statistically significant differences between the groups (p<0.05). The results of the analysis of variance are presented in Table 2.
Table 2. Clinical and laboratory characteristics of patients with PsA in relation to the applied therapy
| Parameter | І group(n = 46) | ІІ group(n = 124) | III group(n=28) | p |
|---|---|---|---|---|
| Duration of Illness, years | 14.2±1.1 | 15.4±0.9 | 17.2±1.3 | p>0.05 |
| Average Age, years | 37.2±2.5 | 39.9±2.1 | 38.4±1.7 | p>0.05 |
| DAPSA, index | 22.14±1.12 | 17.80±1.22 | 14.83±1.37 | p<0.001 |
| PASI, index | 18.7±1.8 | 14.2±0.7 | 11.2±1.2 | p<0,001 |
| C-reactive Protein, mg/L | 9.1±1.19 | 5.2±0.44 | 3.1±0.11 | p<0.001 |
| ESR, mm/hour | 22.3±2.53 | 15.5±1.17 | 11.8±1.32 | p<0.001 |
| QRISK-3, index | 12.1±0.75 | 8.9±0.22 | 7.1±0.31 | p<0.001 |
| Pain by VAS, mm | 75.4±3.5 | 71.1±3.4 | 74.9±2.2 | p>0.05 |
| PsACI, index | 8.9±0.37 | 8.8±0.41 | 9.4±0.42 | p>0.05 |
| CCI, index | 1.45±0.08 | 1.59±0.12 | 1.68±0.18 | p>0.05 |
| HAQ-DI, index | 1.71±0.12 | 1.87±0.15 | 1.84±0.09 | p>0.05 |
| Total cholesterol, mmol/l | 4.95±0.15 | 5.28±0.12 | 5.51±0.17 | p<0.001 |
| TCIM artery, mm | 0.94±0.03 | 1.06±0.02 | 1.15±0.02 | p=0.005 |
Notes: p — significance among patients depending on the received therapy; QRISK-3 is a risk calculator used to assess the likelihood of cardiovascular events, such as heart attacks and strokes, based on various risk factors; VAS — visual analog scale. Source: compiled by the authors
Significant intergroup differences were observed for indicator of inflammatory processes, such as the levels of CRP (p<0.001), disease activity index — DAPSA (p<0.001), severity of skin involvement — PASI (p<0.001), and CV risk based on Q-risk (p<0.001). It is worth noting that no intergroup differences were found for comorbidity indexes. This could be attributed to the fact that the majority of patients did not receive immunobiological and disease-modifying medications from the onset of the disease, but rather after a certain period, when psoriasis had already negatively affected organs and systems.
Upon further post hoc analysis of the data, it became evident that the majority of differences between groups were observed in patients who did not receive any form of treatment (Group I) and those who were treated with DMARDs plus biological therapy (Group III). Significant correlations were identified in bivariate analysis between the outcomes of PsACI and those of various other comorbidity scores and scales, including the CCI (p<0.001, rho=0.852), DAPSA (p<0.001, rho=0.712), PASI (p<0.001, rho=0.421), HAQ (p<0.001, rho=0.387). These findings imply that higher disease activity is associated with a greater comorbidity burden in PsA patients. Furthermore, Spearman’s rank correlation analysis revealed a significant correlation between PsA disease activity and CV risk. The correlation coefficient between DAPSA scores and 10-year CV risk, as estimated by the QRISK-3 scale, was 0.571 (p < 0.001). This finding underscores the link between active PsA disease and increased CV risk in the study population.
Particularly noteworthy is the investigation into the combination of comorbid pathology among individuals of a rheumatological profile, as it significantly worsens the prognosis for patients. Since 2000, numerous studies have been conducted to explore the coexisting medical conditions in people with rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), ankylosing spondylitis (AS) [25-27]. However, research focusing on PsA has been relatively scarce, as it was previously considered a relatively mild disease. Recent studies S.H. Lam et al. [28] have highlighted the potential for psoriatic arthritis to place a significant financial strain on individuals and their families, particularly in relation to cardiovascular conditions like ischemic heart disease, angina, myocardial infarction, transient ischemic attack, peripheral artery disease, stroke, and congestive heart failure. In recent years, extensive research conducted under the guidance of L.M. Perez-Chada & J.F. Merola [9] and S. Gupta et al. [3] has provided comprehensive insights into the associations, predictive capabilities, and correlations between disease activity, comorbidity burden, and CV risk in PsA patients. The results of this study are in concordance with these well-established literature findings, further reinforcing the existing body of knowledge.
Furthermore, this study contributes novel insights by establishing a clear relationship between comorbidity burden, the extent of cutaneous involvement, and functional impairments. These quantitative insights contribute to a comprehensive understanding of the multifaceted nature of psoriatic arthropathy and its implications for patient management and treatment strategies. The use of comorbidity indexes, such as the CCI and the PsACI, provided valuable insights into the overall health status of PsA patients. While CCI is a widely used index for measuring comorbidities in clinical research, it lacks specificity for PsA and other specific diseases. In contrast, PsACI, specifically designed for PsA patients, demonstrated a higher sensitivity in identifying patients at high risk for comorbidities, considers disease severity, includes a broader range of diseases in its calculation, making it a more comprehensive tool for assessing comorbidity burden. This further supports its potential as an effective clinical tool for early detection and management of comorbidities in PsA patients, taking into account the information presented above, which was considered during the subsequent statistical analyses. The findings of this study, indicating a notably elevated comorbidity index with the use of PsACI, are consistent with recent investigations by Y. El Miedany et al. [21], which reported similar trends in PsA patients.
Previous studies have already indicated a connection between PsA and an increased risk of cardiovascular diseases [25, 29]. In this study, the association has been confirmed, revealing that PsA patients have an intermediate risk of cardiovascular events, which is eight to nine times higher than that of healthy individuals. The elevated risk of major adverse CV events among PsA patients not prescribed DMARDs and biological therapy indicates the potential role of its in reducing CV risk in PsA. These results align with emerging evidence suggesting that those antirheumatic drugs could be instrumental in improving cardiovascular outcomes for PsA patients [30-32]. Research involving individuals with rheumatoid arthritis and psoriasis has shown a reduced incidence of CV events in those who undergo anti-tumor necrosis factor alpha (TNF-α) therapy [33].
Moreover, the study illuminates the intricate web of comorbidities often accompanying PsA. Cardiometabolic comorbidities, including dyslipidemia, hypertension, and obesity, were highly prevalent among the study participants. These conditions are known to exacerbate the severity and progression of PsA. The results align with previous research indicating a strong link between PsA and these comorbidities [34]. Additionally, a significant association was found between high disease activity, duration, and functional limitations in PsA and the number of reported comorbidities. This suggests that optimal control and management of PsA disease activity may have a positive impact on comorbidity burden.
CONCLUSIONS
The study aimed to investigate the prevalence and impact of comorbidities in patients with PsA and assess the associations between disease and various comorbid conditions. The analysis revealed a clear predilection for CM comorbidities in examined patients, with CV disorders being the most prevalent, affecting 55.6% of cases. There was also a significant association found between high disease activity, disease duration, and functional limitations in PsA patients and the number of reported comorbidities, emphasizing the importance of considering and managing comorbidities in the clinical management of psoriatic arthropathy.
Furthermore, this research unveiled an elevated risk of cardiovascular events among PsA patients, emphasizing the critical need for targeted interventions to mitigate this risk. The study also identified differences in treatment approaches, with the utilization of DMARDs and TNF-α inhibitors being potential factors in reducing comorbidity burden and CV risk in those patients. Findings emphasize the significance of addressing and effectively managing comorbidities in the clinical care of individuals with psoriatic arthropathy. The study’s limitations stem from its cross-sectional design, preventing the establishment of a causal relationship between psoriatic arthropathy and comorbidities. Nevertheless, the research offers valuable insights into the comorbidity patterns among PsA patients, underscoring the significance of holistic disease management to enhance long-term outcomes and overall quality of life.
Despite the clinical relevance of comorbidities, there is currently a lack of comprehensive research on comorbidities in PsA. This highlights the importance of further investigations to fill this gap and refine understanding of PsA and its associated conditions. However, it’s worth noting that the novelty of this coefficient also introduces certain limitations in its application, as there is currently a lack of clear interpretation of the percentage survival over the next 10 years. Moving forward, research should prioritize further validation of the PsACI in larger, multicenter studies to establish its reliability for assessing comorbidities in PsA patients.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
REFERENCES
- 1. Singh J.A., Guyatt G., Ogdie A. et al. (2019) 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol., 71(1): 5–32.
- 2. Ocampo D.V., Gladman D. (2019) «Psoriatic arthritis.» F1000Research 8: F1000 Faculty Rev-1665.
- 3. Gupta S., Syrimi Z., Hughes D.M. et al. (2021) Comorbidities in psoriatic arthritis: A systematic review and meta-analysis. Rheumatol. Int. 41(2): 275–84.
- 4. Haddad A., Saliba W., Lavi I. et al. (2022) The association of psoriatic arthritis with all-cause mortality and leading causes of death in psoriatic arthritis. J. Rheumatol 49(2): 165–70.
- 5. Elalouf O., Muntyanu A., Polachek A. et al. (2020) Mortality in psoriatic arthritis: Risk, causes of death, predictors for death. Semin. Arthritis Rheum. 50(4): 571–75.
- 6. Khimion L.V., Boiko AV. (2020) Features of the interconnection of traditional risk factors and IL-10 with the activity of the inflammatory process and atherosclerosis development in patients with psoriatic arthritis. Wiad Lek., 73(5): 914–19.
- 7. Marushchak M., Kozak K., Krynytska I. (2022) Comorbid overweight/obesity and chronic pancreatitis exacerbate dyslipidemia progression in type 2 diabetic patients. Endocr. Regul., 56(3): 168–77.
- 8. Babalic F.C.A., Borza C., Ilie Rosca C. et al. (2022) Endothelial dysfunction in psoriatic arthritis patients: Correlations between insulin resistance and disease activity. Eur. Rev. Med. Pharmacol. Sci., 26(18): 6796–4.
- 9. Perez-Chada L.M., Merola J.F. (2020) Comorbidities associated with psoriatic arthritis: Review and update. Clin. Immunol., 214: 108397.
- 10. Ortolan A., Lorenzin M., Tadiotto G. et al. (2019) Metabolic syndrome, non-alcoholic fatty liver disease and liver stiffness in psoriatic arthritis and psoriasis patients. Clin. Rheumatol., 38(10): 2843–50.
- 11. Tejada B., Joehanes R., Hwang S.J. et al. (2022) Systemic inflammation is associated with cardiometabolic risk factors and clinical outcomes. J. Inflamm. Res., 15: 6891–3.
- 12. Coates L.C., Corp N., van der Windt D.A. et al. (2022) GRAPPA treatment recommendations: 2021 Update. J. Rheumatol., 49(6 Suppl. 1): 52–54.
- 13. Taylor W., Gladman D., Helliwell P. et al.; CASPAR Study Group. (2006) Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum., 54(8): 2665–73.
- 14. Welcome to the QRISK®3 risk calculator [Internet] [cited 2023 Oct 6]. Available from: http://www.qrisk.org.
- 15. Smiyan S., Bilukha A. (2021) Using q-risk scale for checking cardiovascular risk in patients with psoriatic arthritis. Ann. Rheum. Dis., 80(Suppl. 1): 1320–21.
- 16. Calculate the 10-year risk of fatal and non-fatal cardiovascular disease events of your patients: Heartscore [Internet] [cited 2023 Oct 6]. Available from: http://www.heartscore.org.
- 17. Yoshii I., Chijiwa T., Sawada N. (2018) Influence of pain score measured by a visual analog scale (PS-VAS) on the Health Assessment Questionnaire Disability Index and 28-joint Disease Activity Index with C-reactive protein in rheumatoid arthritis patients. Int. J. Rheum. Dis., 21(11): 1955–61.
- 18. Ritchlin C.T., Colbert R.A., Gladman D.D. (2017) Psoriatic Arthritis. N. Engl. J. Med., 376(10): 957–70.
- 19. Gudu T., Gossec L. (2018) Quality of life in psoriatic arthritis. Expert Rev. Clin. Immunol., 14(5): 405–17.
- 20. Charlson M.E., Pompei P., Ales K.L. et al. (1987) A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis., 40(5): 373–83.
- 21. El Miedany Y., El Gaafary M., Youssef S. et al. (2017) Psoriatic arthritis comorbidity index: development and validation of a new specific tool for classifying prognostic comorbidity in psoriasis and psoriatic arthritis patients. Rheumatol. Orthop. Med., 2(2): 1–7.
- 22. Charlson M.E., Carrozzino D., Guidi J. et al. (2022) Charlson comorbidity index: A critical review of clinimetric properties. Psychother. Psychosom., 91(1): 8–35.
- 23. Popescu N.D., Dima A., Berza I.A. et al. (2022) Concordance between different comorbidities scores in patients with psoriatic arthritis. Ann. Rheum. Dis., 81: 1605–6.
- 24. The World Medical Association (Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects [Internet]. Available from: http://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/.
- 25. Smiyan S.I., Koshak B.O., Gnatko I.V. (2017) Endothelial dysfunction and cardiovascular risk in patients with ankylosing spondylitis. Int. J. Med. Res.; 3 (Iss. 2): 5–9.
- 26. Figus F.A., Piga M., Azzolin I. et al. (2021) Rheumatoid arthritis: Extra-articular manifestations and comorbidities. Autoimmun. Rev., 20(4): 102776.
- 27. Smiyan S., Koshak B., Komorovsky R. et al. (2023) Diagnostic challenge of tuberculosis in systemic lupus erythematosus: A case report and literature review. Rheumatol. Int., 43(11): 2131–39.
- 28. Lam S.H., So H., Cheng I.T. et al. (2021) Association of C-reactive protein and non-steroidal anti-inflammatory drugs with cardiovascular events in patients with psoriatic arthritis: A time-dependent Cox regression analysis. Ther. Adv. Musculoskelet. Dis., 13: 1759720X211027712.
- 29. Ramírez J., Azuaga-Piñango A.B., Celis R. et al. (2021) Update on cardiovascular risk and obesity in psoriatic arthritis. Front. Med. (Lausanne), 8: 742713.
- 30. Sparks J.A., Lesperance T., Accortt N.A. et al. (2019) Subsequent cardiovascular events among patients with rheumatoid arthritis, psoriatic arthritis, or psoriasis: Patterns of disease-modifying antirheumatic drug treatment. Arthritis Care Res. (Hoboken), 71(4): 512–20.
- 31. Gossec L., Baraliakos X., Kerschbaumer A. et al. (2020) EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis., 79(6): 700–12.
- 32. Ayan G., Ribeiro A., Macit B. et al. (2023) Pharmacologic treatment strategies in psoriatic arthritis. Clin. Ther., 45(9): 826–40.
- 33. Zheng Z., Guo Q., Ma D. et al. (2022) Related risk factors and treatment management of psoriatic arthritis complicated with cardiovascular disease. Front. Cardiovasc. Med., 9: 835439.
- 34. Panagiotopoulos A., Fragoulis G.E. (2023) Comorbidities in psoriatic arthritis: A narrative review. Clin. Ther. 45(2): 177–89.
Адреса для листування:
Тернопільський національний медичний університет ім. І.Я. Горбачевського МОЗ України
Кафедра функціональної та лабораторної діагностики
46000, м. Тернопіль, вул. Клінічна, 1

Leave a comment